Our solutions
At XEMPERIA, we apply cutting-edge innovations to redefine solutions for early cancer detection, monitoring and therapy selection, providing patients and physicians with vital information at all stages of the disease.
Our Breast Cancer Screening Blood Test. XEMPERIA is preparing a large-scale performance study to certify its breast cancer screening test. The final product will consist of a PCR kit compatible with standard IVD qPCR equipment, along with an algorithm for data interpretation. Market entry is targeted for 2028, supported by a combination of private investment and public grants.
Our Nanosensors for Nucleic Acid Detection (DNA origami nanotechnology for microRNA detection and Nanosensor for mutation detection). Initial commercial development of these cutting-edge technologies will focus on Research Use Only (RUO) applications for rapid, sensitive, and precise detection of miRNAs and DNA mutations. The project is being carried out in collaboration with the University of Fribourg and is funded by competitive research grants. RUO market entry is expected between 2026 and 2027. The development of a clinical-grade test is planned for the next phase.
XEMPERIA is actively advancing the commercialization of its innovative solutions.
ROADMAP
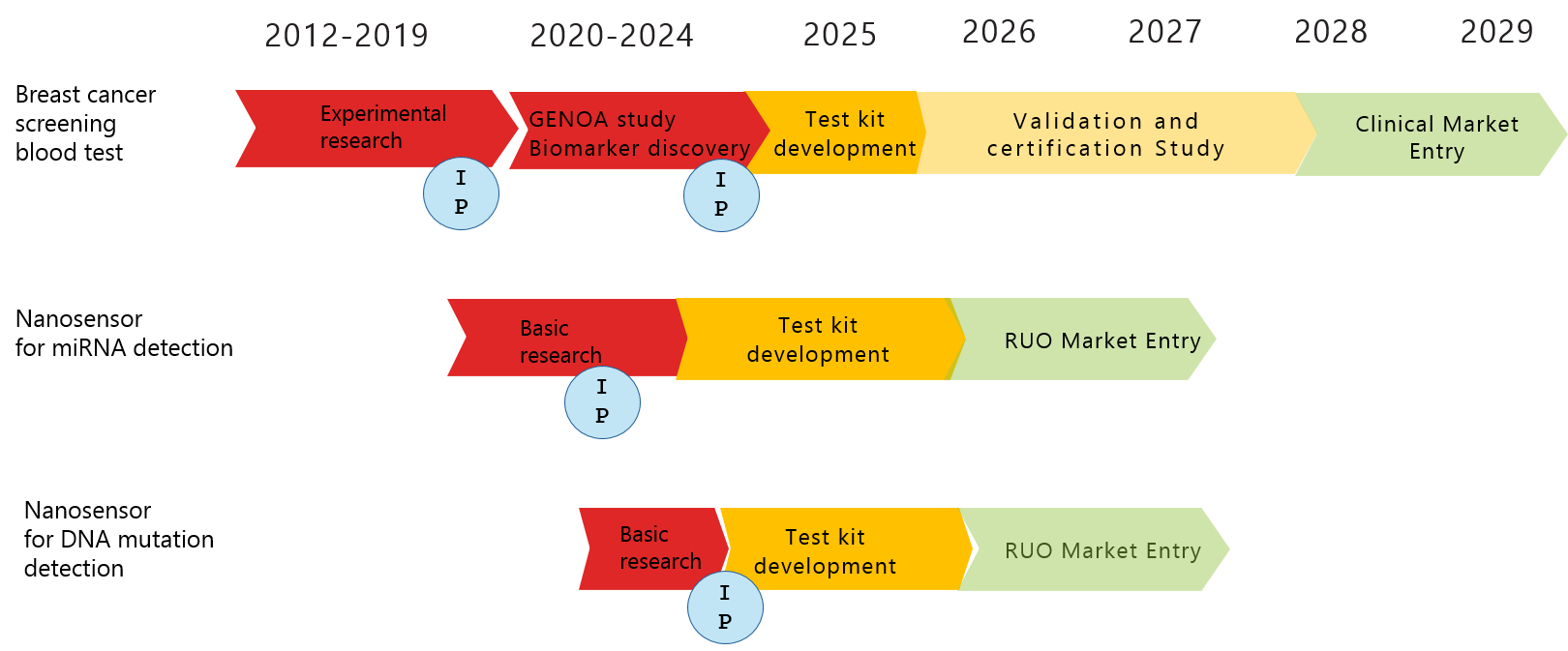
Our technologies are all intellectually protected (IP).
Our technologies are evolutive and adaptable to address additional clinical needs:
● Stratify high- vs low-risk patients. Helping doctors choose the best therapy from the start.
● Patient monitoring. Rapidly detect progression and therapy adaptation.
● Detection of many kind of cancer. Early detection of other cancers that can benefit from early treatment.
● Monitorng patients with chronic conditions. These include inflammatory,
cardiovascular, neurological diseases.
Interested in knowing more?
- © 2025 Xemperia. All rights reserved | Imprint | Design: ZebraZbinden | Development: FRESCHI IT